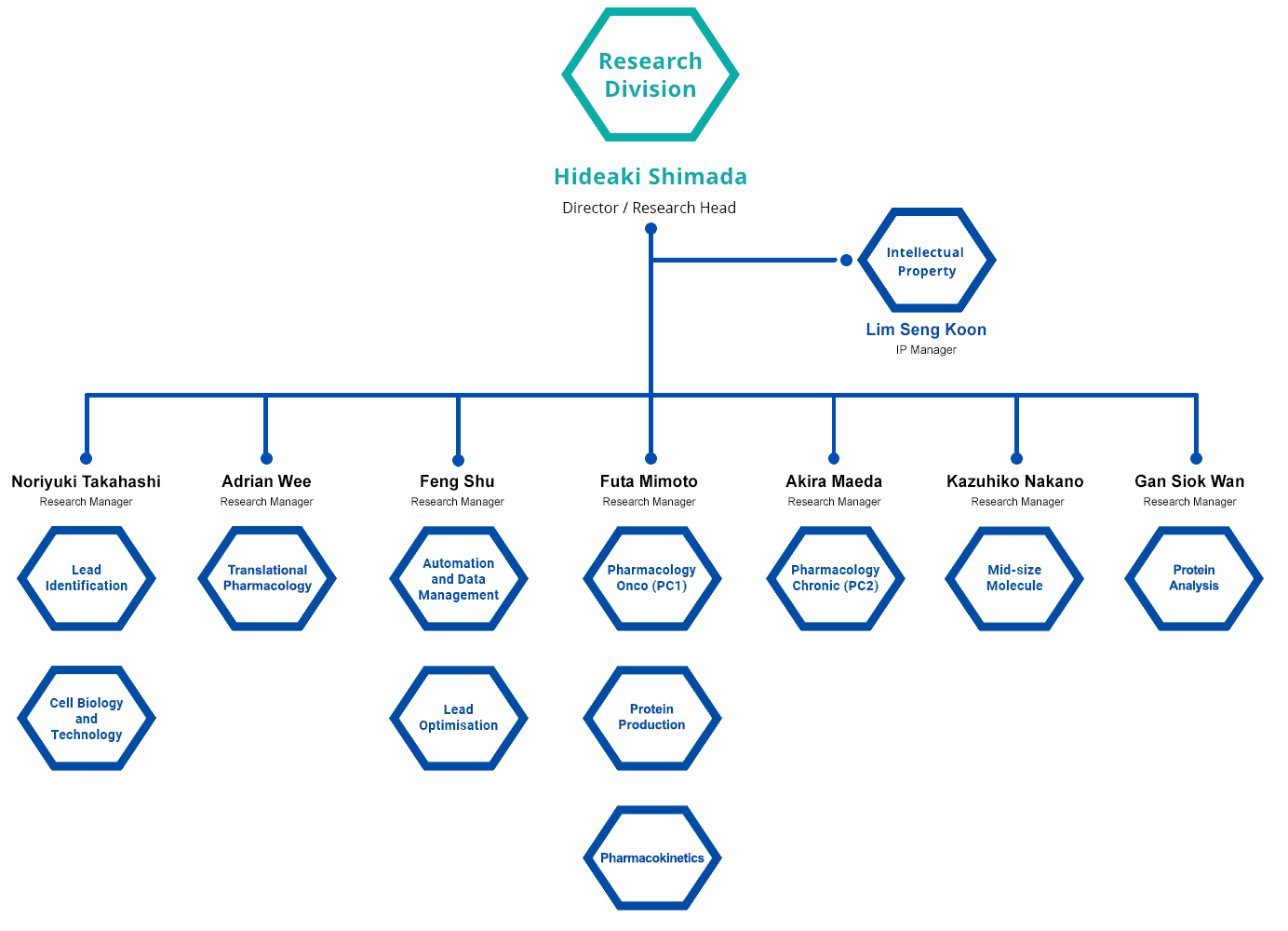
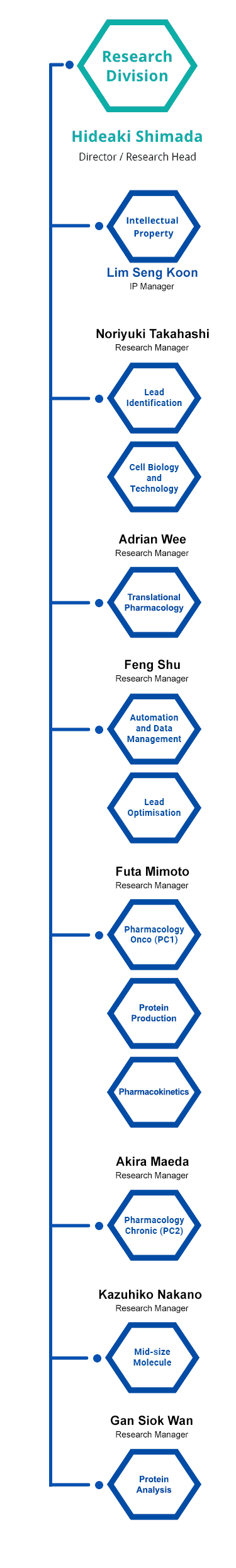
Research Units
There are 10 research units at CPR. These units have independent functions in antibody research and development, and they collaborate as one to accelerate the drug discovery process.
Lead Identification Unit
The Lead Identification (LI) unit drives discovery and identification of high-quality therapeutic antibody candidates and antibodies required by other units for assay systems and proof-of-concept studies. To achieve maximum output of lead antibodies, high throughput systems significantly influence selection and screening processes. The LI unit is equipped with flow cytometers for high-speed sorting, automation workstations, and liquid handling systems to accelerate workflow, allowing for thousands of antibodies to be screened in a short duration of time.
Automation and Data Management Unit
The Automation Unit (AU) integrates multiple high-throughput platforms, including plasmid generation, protein mutagenesis and production, and multi-dimensional sample analyses, to improve CPR’s research productivity. The AU also provides data generation and integration solutions for all units, transforming data into intelligence with user-friendly interfaces and accelerated research.
Translational Pharmacology Unit
The Translational Pharmacology (TP) unit is responsible for immunisation, PK/PD analysis and biomarker exploration, and pharmacological study support.
Protein Production Unit
The Protein Production (PP) unit is dedicated to producing antibody and non-antibody proteins of a high caliber. Our activities include transient expression of recombinant proteins using mammalian and bacterial systems, and purification of recombinant and endogenous proteins with various types of column chromatography. Efficient protein production by the PP unit drives drug discoveries in CPR.
Lead Optimisation Unit
The Lead Optimisation (LO) unit is the chief division in steering lead antibody drugs to meet the target antibody profile. Through comprehensive mutagenesis and high throughput screening, the unit identifies monoclonal antibodies with vastly improved binding characteristics, physicochemical and pharmacokinetic properties, and minimal immunogenicity. This antibody engineering process encompasses molecular cloning, small-scale protein expression in mammalian system and multi-dimensional analysis. They work closely with other CPR research units to discover and optimize suitable drug candidates.
Protein Analysis Unit
The mission of Protein Analysis (PA) unit is to reveal the mechanism and to assess developability of antibodies. We confer unique properties on antibodies though fine-tune engineering. Therefore, it is critical for the success of our clinical candidate selection to understand how these highly engineered antibodies work. The PA unit reveal the critical properties of antibodies utilizing their expertise of various analytical equipment: HPLC (High-performance liquid chromatography), LC-MS (Liquid chromatography–mass spectrometry), and more.
Pharmacology Unit
The Pharmacology (PC) unit is the central body in CPR responsible for establishing and optimizing assays to evaluate candidate antibodies. They prioritize on designing assays which are not only robust and sensitive, but also physiologically relevant to the natural biology of target molecules. In addition, the unit is tasked with screening panels of antibodies to identify those with superior characteristics. To achieve this, they employ high-throughput assay systems to assess antibody functions generated by other research units within CPR. Thus, the PC unit strongly values broad expertise in multiple areas not limited to pharmacology, molecular biology, biochemistry, physiology, and pathology.
Mid-size Molecule Unit
In addition to accelerating drug discovery using its proprietary antibody technologies, Chugai Pharmaceutical Co., Ltd. (Chugai) has been establishing new modality discovery technologies for more than a decade. Mid-size molecules, particularly, have significant potential in targeting diseases that are difficult for antibodies and small molecules to target. So, Chugai transferred this discovery technology to CPR with researchers who possess industry-leading research and technological capabilities back by chemical biology and biotechnology.
With the Mid-size Molecule (MM) unit beginning operations in October 2018, CPR’s researchers have focused on improving this modality and developing a strong team locally by transferring knowledge and experience from Japanese to local researchers. To tackle incurable diseases, researchers with multiple expertise in chemical biology, biotechnology, robotics, and bioinformatics among other fields are required to engineer innovative Mid-size molecule technologies.
Cell Biology and Technology unit
The Cell Biology and Technology (CT) unit was established in April 2021 to expand CPR’s research capabilities. They carry out new research concepts that aim to cure or prevent illnesses beyond treatment by taking a regenerative approach among others. 3D organoids and spheroids are established and used for in vitro functional assays and molecule screenings for novel therapeutic candidates. Despite the challenges, high content imagers, flow cytometers, and other lab instruments help us to elucidate molecular spatial profiles, cellular dynamics, and cell differentiation.
Pharmacokinetics Unit
The Pharmacokinetics (PK) unit plays a critical role in antibody engineering by setting the goal of target antibody profile. Controlling antibody pharmacokinetics is the key for various antibody engineering technologies like tumor- and tissue-specific antibody delivery. To serve this mission, the PK unit analyzes the in vivo antibody concentration change over time and target engagement using various analytical methods (e.g. ligand binding assay, western blot, immuno-precipitation, multiplex analysis, qPCR). In addition, PK unit sets target antibody profiles through model and simulation, connecting different properties of antibodies with its in vivo pharmacokinetic profile. This provides insight on how each property can be altered to attain an optimized target antibody profile.
The Lead Identification (LI) unit drives discovery and identification of high-quality therapeutic antibody candidates and antibodies required by other units for assay systems and proof-of-concept studies. To achieve maximum output of lead antibodies, high throughput systems significantly influence selection and screening processes. The LI unit is equipped with flow cytometers for high-speed sorting, automation workstations, and liquid handling systems to accelerate workflow, allowing for thousands of antibodies to be screened in a short duration of time.
The Automation Unit (AU) integrates multiple high-throughput platforms, including plasmid generation, protein mutagenesis and production, and multi-dimensional sample analyses, to improve CPR’s research productivity. The AU also provides data generation and integration solutions for all units, transforming data into intelligence with user-friendly interfaces and accelerated research.
The Translational Pharmacology (TP) unit is responsible for immunisation, PK/PD analysis and biomarker exploration, and pharmacological study support.
The Lead Optimisation (LO) unit is the chief division in steering lead antibody drugs to meet the target antibody profile. Through comprehensive mutagenesis and high throughput screening, the unit identifies monoclonal antibodies with vastly improved binding characteristics, physicochemical and pharmacokinetic properties, and minimal immunogenicity. This antibody engineering process encompasses molecular cloning, small-scale protein expression in mammalian system and multi-dimensional analysis. They work closely with other CPR research units to discover and optimize suitable drug candidates.
The Protein Production (PP) unit is dedicated to producing antibody and non-antibody proteins of a high caliber. Our activities include transient expression of recombinant proteins using mammalian and bacterial systems, and purification of recombinant and endogenous proteins with various types of column chromatography. Efficient protein production by the PP unit drives drug discoveries in CPR.
The mission of Protein Analysis (PA) unit is to reveal the mechanism and to assess developability of antibodies. We confer unique properties on antibodies though fine-tune engineering. Therefore, it is critical for the success of our clinical candidate selection to understand how these highly engineered antibodies work. The PA unit reveal the critical properties of antibodies utilizing their expertise of various analytical equipment: HPLC (High-performance liquid chromatography), LC-MS (Liquid chromatography–mass spectrometry), and more.
The Pharmacology (PC) unit is the central body in CPR responsible for establishing and optimizing assays to evaluate candidate antibodies. They prioritize on designing assays which are not only robust and sensitive, but also physiologically relevant to the natural biology of target molecules. In addition, the unit is tasked with screening panels of antibodies to identify those with superior characteristics. To achieve this, they employ high-throughput assay systems to assess antibody functions generated by other research units within CPR. Thus, the PC unit strongly values broad expertise in multiple areas not limited to pharmacology, molecular biology, biochemistry, physiology, and pathology.
In addition to accelerating drug discovery using its proprietary antibody technologies, Chugai Pharmaceutical Co., Ltd. (Chugai) has been establishing new modality discovery technologies for more than a decade. Mid-size molecules, particularly, have significant potential in targeting diseases that are difficult for antibodies and small molecules to target. So, Chugai transferred this discovery technology to CPR with researchers who possess industry-leading research and technological capabilities back by chemical biology and biotechnology.
With the Mid-size Molecule (MM) unit beginning operations in October 2018, CPR’s researchers have focused on improving this modality and developing a strong team locally by transferring knowledge and experience from Japanese to local researchers. To tackle incurable diseases, researchers with multiple expertise in chemical biology, biotechnology, robotics, and bioinformatics among other fields are required to engineer innovative Mid-size molecule technologies.
The Cell Biology and Technology (CT) unit was established in April 2021 to expand CPR’s research capabilities. They carry out new research concepts that aim to cure or prevent illnesses beyond treatment by taking a regenerative approach among others. 3D organoids and spheroids are established and used for in vitro functional assays and molecule screenings for novel therapeutic candidates. Despite the challenges, high content imagers, flow cytometers, and other lab instruments help us to elucidate molecular spatial profiles, cellular dynamics, and cell differentiation.
The Pharmacokinetics (PK) unit plays a critical role in antibody engineering by setting the goal of target antibody profile. Controlling antibody pharmacokinetics is the key for various antibody engineering technologies like tumor- and tissue-specific antibody delivery. To serve this mission, the PK unit analyzes the in vivo antibody concentration change over time and target engagement using various analytical methods (e.g. ligand binding assay, western blot, immuno-precipitation, multiplex analysis, qPCR). In addition, PK unit sets target antibody profiles through model and simulation, connecting different properties of antibodies with its in vivo pharmacokinetic profile. This provides insight on how each property can be altered to attain an optimized target antibody profile.
Organisation of Research Units